The fight against climate change might be gaining pace, but it seems green energy silicon solar cells are reaching their limits. The most direct way to make the conversion right now is with solar panels, but there are other reasons why they’re the great hope of renewable energy.
Their key component, silicon, is the second most abundant substance on Earth after oxygen. Since panels can be put where the power is needed – on homes, factories, commercial buildings, ships, road vehicles – there’s less need to transmit power across landscapes; and mass production means solar panels are now so cheap the economics of using them are becoming inarguable.
According to the International Energy Agency’s 2020 energy outlook report, solar panels in some locations are producing the cheapest commercial electricity in history.
Even that traditional bug-bear “what about when it’s dark or cloudy?” is becoming less problematic thanks to transformative advances in storage technology.
Moving beyond the limits of solar
If you’re expecting a “but”, here it is: but silicon solar panels are reaching the practical limits of their efficiency because of some quite inconvenient laws of physics. Commercial silicon solar cells are now only about 20 percent efficient (though up to 28 percent in lab environments. Their practical limit being 30 percent, meaning they can only ever convert about a third of the Sun’s received energy into electricity).
Still, a solar panel will produce many times more emissions-free energy in its lifetime than was used in its manufacture.
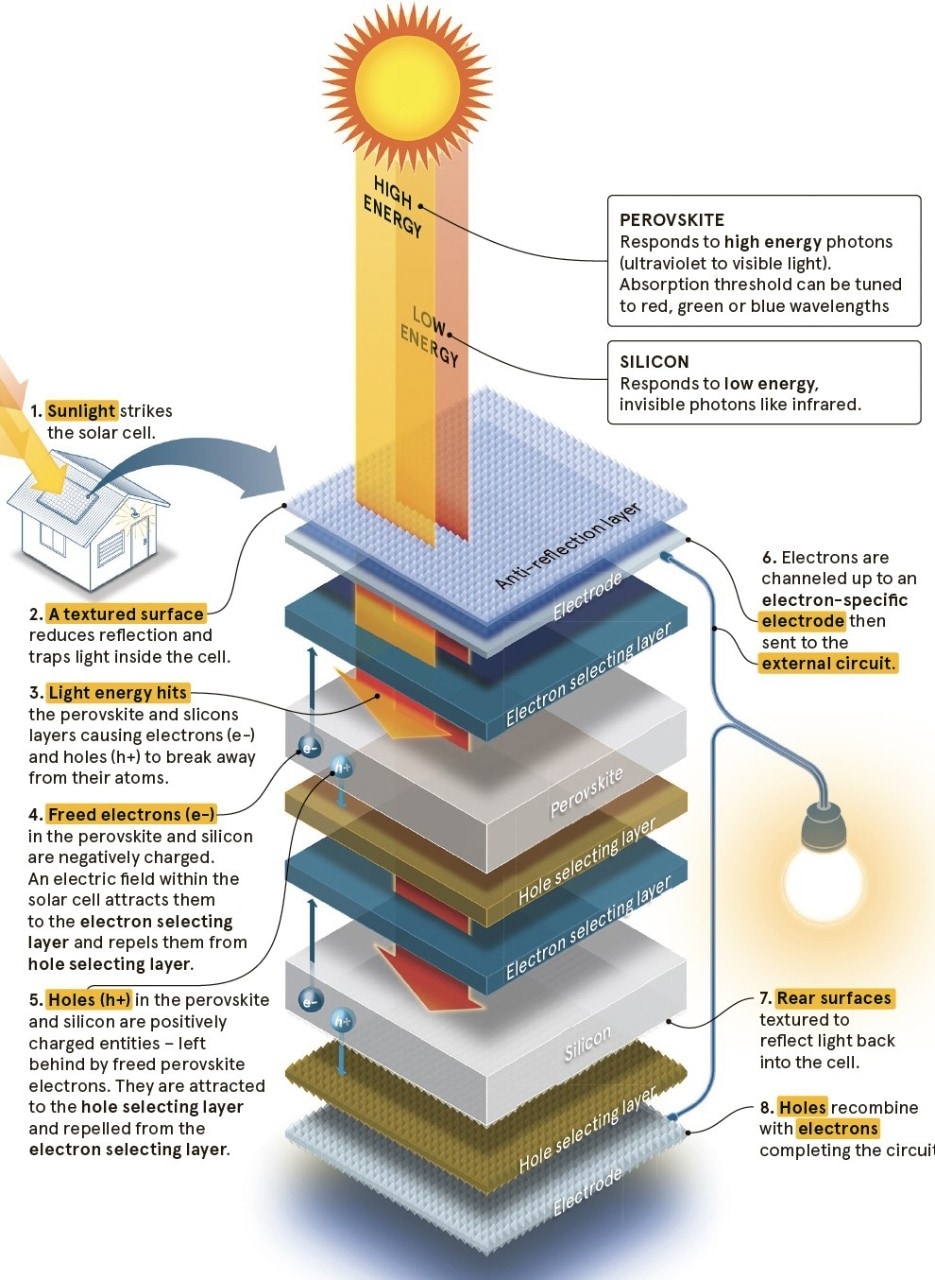
a silicon/perovskite solar cell
Perovskite: the future of renewables
Like silicon, this crystalline substance is photoactive, meaning that when it’s hit by light, electrons in its structure become excited enough to break away from their atoms (this freeing of electrons is the basis of all electricity generation, from batteries to nuclear power plants). Given that electricity is in effect, a conga line of electrons, when the loose electrons from silicon or perovskite are channelled into a wire, electricity is the result.
Perovskite is a simple mixture of salt solutions that is heated to between 100 and 200 degrees to establish its photoactive properties.
Like ink, it can be printed onto surfaces, and it’s bendable in a way that rigid silicon isn’t. Being used at a thickness of up to 500 times less than silicon, it’s also super-light and can be semi-transparent. This means it can be applied to all sorts of surfaces like on phones and windows. The real excitement though, is around perovskite’s energy production potential.
Overcoming perovskite’s biggest challenge – deterioration
The first perovskite devices in 2009 converted just 3.8 percent of sunlight into electricity. By 2020, efficiency was 25.5 percent, close to silicon’s lab record of 27.6 percent. There is a sense that its efficiency could soon reach 30 percent.
If you’re expecting a ‘but’ about perovskite, well, there is a couple. A component of the perovskite crystalline lattice is lead. The quantity is tiny, but the potential toxicity of lead means it is a consideration. The real problem is that unprotected perovskite easily degrades through heat, moisture and humidity, unlike silicon panels which are routinely sold with 25-year guarantees.
Silicon is better at dealing with low-energy light waves, and perovskite works well with higher-energy visible light. Perovskite can also be tuned to absorb different wavelengths of light – red, green, blue. With careful aligning of silicon and perovskite, this means each cell will turn more of the light spectrum into energy.
The numbers are impressive: a single layer could be 33 percent efficient; stack two cells, it’s 45 percent; three layers would give 51 percent efficiency. These sorts of figures, if they can be realised commercially, would revolutionise renewable energy.
Post time: Aug-12-2021